Analysis by Dr. Joseph Mercola
Story at-a-glance
- Moderna and Pfizer’s COVID-19 vaccines use lipid nanoparticles that contain polyethylene glycol (PEG) to deliver mRNA to your cells
- This experimental mRNA technology and its lipid nanoparticle-based delivery system have never been approved for use in a vaccine or drug
- Significant concerns have been raised over the technology, including the lipid nanoparticles, and Moderna actually abandoned it in 2017 after studies revealed a high rate of adverse effects
- Experts have questioned whether LNPs are safe, as research shows they readily enter the brain and may trigger immune reactions
- The first dose of COVID-19 vaccine may prime the body to have an immune reaction to LNPs, such that when the second dose is received, a stronger likelihood for adverse events exists
- The PEG found in both Pfizer’s and Moderna’s vaccines could also be causing allergic reactions and anaphylaxis
Both the Pfizer-BioNTech and Moderna COVID-19 vaccines are made with messenger RNA (mRNA) technology, which works by turning your body’s cells into veritable drug factories. The mRNA vaccines teach your cells to produce a protein, or piece of protein, that triggers an immune response, including the production of antibodies.1
However, because natural mRNA is easily broken down, this means the experimental gene therapy needs a special delivery system to make it to the body’s cells.
Moderna and Pfizer are using lipid nanoparticles that contain polyethylene glycol (PEG)2 for this purpose. The mRNA is wrapped in lipid nanoparticles (LNPs) that carry it to your cells, and the LNPs are “PEGylated” — that is, chemically attached to PEG molecules to increase stability.3
This experimental mRNA gene therapy and its lipid nanoparticle-based delivery system have never been approved for use in a vaccine or drug.4 This includes Pfizer’s and Moderna’s COVID-19 vaccines, which were only “authorized” for emergency use by the U.S. Food and Drug Administration — not “approved.”5
Significant concerns have been raised over the technology, including the lipid nanoparticles, and Moderna actually abandoned it in 2017 after studies revealed a high rate of adverse effects.
Moderna Abandoned mRNA Project in 2017
Moderna partnered with the National Institute of Allergy and Infectious Diseases (NIAID) headed by Dr. Anthony Fauci to create its COVID-19 vaccine. In February 2020, its stock price increased 78.1% when it announced that its messenger RNA vaccine was ready for clinical trials,6 propelling its CEO Stéphane Bancel into the billionaire’s club.
In 2016, Bancel had talked up another of the company’s up-and-coming products, a drug treatment for a rare disease called Crigler-Najjar syndrome. Those with Crigler-Najjar syndrome are missing a liver enzyme needed to break down bilirubin. Moderna’s experimental treatment used mRNA to encode for the missing enzyme, and was encased in LNPs as the delivery agent.
But, as STAT reported in 2017, the treatment was “indefinitely delayed” because it “never proved safe enough to test in humans.”7 According to STAT:8
“… mRNA is a tricky technology. Several major pharmaceutical companies have tried and abandoned the idea, struggling to get mRNA into cells without triggering nasty side effects … The indefinite delay on the Crigler-Najjar project signals persistent and troubling safety concerns for any mRNA treatment that needs to be delivered in multiple doses …
… And for its chemists, those nanoparticles created a daunting challenge: Dose too little, and you don’t get enough enzyme to affect the disease; dose too much, and the drug is too toxic for patients … Moderna could not make its therapy work, former employees and collaborators said. The safe dose was too weak, and repeat injections of a dose strong enough to be effective had troubling effects on the liver in animal studies.”
Moderna Says LNPs Could Trigger ‘Significant Adverse Events’
In their corporate prospectus9 released in 2018 at the time of their stock market launch, Moderna acknowledged that their LNPs carried risks.10 They stated:11
“No mRNA drug has been approved in this new potential category of medicines, and may never be approved as a result of efforts by others or us. mRNA drug development has substantial clinical development and regulatory risks due to the novel and unprecedented nature of this new category of medicines.”
They also laid out the many potential risks related to mRNA or its LNP delivery system. Among them:
- “Gene therapies and mRNA based medicines may activate one or more immune responses against any and all components of the drug product (e.g., the mRNA or the delivery vehicle, such as a lipid nanoparticle (LNP)) as well as against the encoded protein, giving rise to potential immune reaction related adverse events.”12
- “Most of our investigational medicines are formulated and administered in an LNP which may lead to systemic side effects related to the components of the LNP which may not have ever been tested in humans.”13
- “While we have continued to optimize our LNPs, there can be no assurance that our LNPs will not have undesired effects. Our LNPs could contribute, in whole or in part, to one or more of the following: immune reactions, infusion reactions, complement reactions, opsonation reactions, antibody reactions including IgA, IgM, IgE or IgG or some combination thereof, or reactions to the PEG from some lipids or PEG otherwise associated with the LNP.”14
- “Certain aspects of our investigational medicines may induce immune reactions from either the mRNA or the lipid as well as adverse reactions within liver pathways or degradation of the mRNA or the LNP, any of which could lead to significant adverse events in one or more of our clinical trials.”15
LNPs May Enter Your Brain
In a letter to Dr. Peter Marks,16 director of the FDA’s Center for Biologics Evaluation and Research, the Informed Consent Action Network (ICAN) questioned whether LNPs are safe, as research shows they readily enter the brain:
“A 2018 study titled Lipid Nanoparticles: A Novel Approach for Brain Targeting17 states, ‘… lipid nanoparticles are taken up readily by the brain because of their lipophilic nature. The bioacceptable and biodegradable nature of lipid nanoparticles makes them less toxic and suited for brain targeting.’ The article also states, ‘these nanostructures need to be investigated intensively to successfully reach the clinical trials stage.'”
The study’s authors suggested lipid nanoparticles like those in COVID-19 vaccines may be ideal for drug delivery systems because of their ability to bypass the blood brain barrier and “reach the target site due to their small size and ability to dodge the reticular endothelial system.”18
I recently interviewed Judy Mikovits, Ph.D., a cellular and molecular biologist, and she agreed that LNPs can enter the brain can contribute to pathologic neuroinflammation, possibly leading to adverse effects like multiple sclerosis or ALS. Additionally, these LNPs carrying the mRNA last for long periods of time, forcing your cells to continuously produce the SARS-CoV-2 spike protein. I encourage you to review my article and interview with Judy.
The mRNA vaccine triggers your body to produce antibodies against the SARS-CoV-2 spike protein, and spike proteins in turn contain syncytin-homologous proteins that are essential for various functions in your body, including the formation of the placenta in pregnant women.
“Syncytin is the name given to the endogenous gamma retrovirus envelope,” Mikovits said, “and we know if it’s expressed overtly in the body in different places … for instance, in the brain, where these lipid nanoparticles will go, then you’ve got multiple sclerosis.” She continued:
“So the expression of that gene alone enrages microglia, literally inflames, and dysregulates the communication between the brain microglia, which are critical for clearing toxins and pathogens in the brain, and the communication with the astrocytes that dysregulates not only the immune system but the endocannabinoid system …
We’ve already seen in the clinical trials … multiple sclerosis as an adverse event, and we’re being lied to. ‘Oh, those people had that.’ No, they didn’t. And we also see myalgic encephalomyelitis, inflammation of the brain and the spinal cord.”
A petition to the European Medicine Agency also called for a halt to Phase 3 clinical trials of Pfizer’s mRNA vaccine, in part due to the concern that if a woman’s immune system starts reacting against syncytin-1, there is the possibility she could become infertile.19
Immune Reactions Are Another Key Concern
According to ICAN in their letter to the FDA,20 the first dose of COVID-19 vaccine may prime the body to have an immune reaction to LNPs, such that when the second dose is received, a stronger likelihood for adverse events exists:
“An article titled Side Effects and COVID-19 Vaccines: What to Expect, published by Johns Hopkins states, ‘Side effects were more frequent after the second dose in the vaccine trials.’21
This event is demonstrated in Exogenous nanoparticles and endogenous crystalline molecules as danger signals for the NLRP3 inflammasomes, which provides support that increasingly inflammatory side-effects observed in the vaccine arm of the trial are attributable to LNPs and that they get worse with repeated injection.
This increased reactogenicity is clearly illustrated in COVID-19 Clinical Trials for both the Pfizer22 and Moderna.”23
Moderna’s report of clinical safety to the FDA’s Vaccines and Related Biological Products Advisory Committee also noted, “In Study 301, solicited local and systemic ARs [adverse reactions] were more common in participants who received mRNA-1273 compared with placebo, and systemic ARs were more common after the second injection.”24
Their data show that Grade 3 systemic adverse reactions, which are severe enough to prevent daily activities, rose from 2.9% after the first dose to 15.7% after the second dose. Grade 4 reactions, which require emergency room visits or hospitalizations, also increased, from five cases after the first injection to 14 cases after the second.25
At least eight people have also had severe allergy-like reactions to Pfizer’s COVID-19 vaccine, prompting NIAID to convene several meetings to discuss the adverse events with officials from Pfizer and Moderna, along with the FDA and independent scientists.26
Many suspect the PEG found in both Pfizer’s and Moderna’s vaccines might be the culprit causing allergic reactions and anaphylaxis. PEG has never been used in an approved vaccine, but is used in certain drugs known to cause anaphylaxis.27 According to Robert F. Kennedy Jr., “studies show that 1 in 7 Americans28 may unknowingly be at risk of experiencing an allergic reaction to PEG.”29
He believes “everyone should be screened for anti-PEG antibodies before getting the Pfizer and Moderna vaccines,” adding that “It is unconscionable that, instead, the FDA and CDC are encouraging people to go ahead and risk a life-threatening anaphylactic reaction and just assume that someone will be on hand to save them.”30
Moderna’s Vaccine Facing Legal Troubles?
It’s also worth noting that Moderna has no legal rights to a key patent for its vaccine delivery system, and company executives are among those who have dumped their stock. Both Moderna and the NIH are essentially engaged in patent infringement, as a core part of the technology — the LNP technology that is part of the vaccine delivery system — belongs to a small Canadian biotech company called Arbutus.31
Moderna sought to invalidate the patent owned by Arbutus Biopharma, but lost the challenge at the end of July 2020.32 After losing the challenge, Moderna said their LNP technology is actually far more advanced than Arbutus’ and claimed “the LNP used to make mRNA-1273, its Covid-19 vaccine candidate, is not covered by the Arbutus patent.”33
“Whatever happens on the intellectual property front, it is highly unlikely that a patent issue will get in the way of the development or distribution of a Covid-19 vaccine,” Forbes noted in July 2020.34
Indeed the vaccine has already been rolled out, but before making your decision on whether or not to receive it, be sure you’re informed about the many unanswered questions and risks surrounding it, including not only those related to LNPs but also another significant issue known as antibody‐dependent enhancement (ADE).
What to Do if You or Someone You Know Received the Vaccine
The primary reason why I wanted to interview Mikovits was to find out her recommendations for those who chose to get the vaccine and now regret it. Interestingly, what I learned is that you would use the same strategies that you would use to treat the actual SARS-CoV-2 infection.
I’ve written many articles over the past year detailing simple strategies to improve your immune system, and with a healthy immune system, you’ll get through it without incident even if you end up getting sick. Below, I’ll summarize some of the strategies you can use both to prevent COVID-19 and address any side effects you may encounter from the vaccine.
First of all, you’ll want to eat a “clean,” ideally organic diet. Avoid processed foods of all kinds, as they are loaded with damaging omega-6 linoleic acid that wrecks your mitochondrial function. Also consider nutritional ketosis and time-restricted eating, both of which will help you optimize your metabolic machinery and mitochondrial function. As noted by Mikovits:
“We have to think about detoxing metal, we have to think about glyphosate … We have to prevent inflammation in all tissue sites and we have to keep our immune system healthy … You’re going to want to be burning ketones instead [of sugar] for the neuroinflammation, so you’re going to want to get into ketosis and take the stress off the mTOR pathway.”
With regard to glyphosate, a simple way to block glyphosate uptake is to take glycine. Approximately 3 grams, about half a teaspoon, a few times a day should be sufficient, along with an organic diet, so that you’re not adding more glyphosate with each meal.
To improve detoxification, I recommend activating your natural glutathione production with molecular hydrogen tablets. All of these strategies should help improve your resilience against SARS-CoV-2, and may even help your body detoxify if you’ve made the mistake of getting this experimental gene therapy.
Another helpful strategy is to maintain a neutral pH. You want your pH to be right around 7, which you can measure with an inexpensive urine strip.
The lower your pH, the more acidic you are. A simple way to raise your pH if it’s too acidic (and most people are) is to take one-fourth teaspoon of sodium bicarbonate (baking soda) or potassium bicarbonate in water a few times a day. Improving your pH will improve the resiliency of your immune system and reduce the mineral loss from your bones, thereby reducing your risk of osteoporosis.
Helpful Supplements
Nutritional supplementation can also be helpful. Among the most important are:
| N-acetylcysteine (NAC) — NAC is a precursor to reduced glutathione, which appears to play a crucial role in COVID-19. According to one literature analysis,35 glutathione deficiency may actually be associated with COVID-19 severity, leading the author to conclude that NAC may be useful both for its prevention and treatment. |
| Zinc — Zinc plays a very important role in your immune system’s ability to ward off viral infections. Like vitamin D, zinc helps regulate your immune function36 — and a combination of zinc with a zinc ionophore, like hydroxychloroquine or quercetin, was in 2010 shown to inhibit SARS coronavirus in vitro. In cell culture, it also blocked viral replication within minutes.37 Importantly, zinc deficiency has been shown to impair immune function.38 |
| Melatonin — Boosts immune function in a variety of ways and helps quell inflammation. Melatonin may also prevent SARS-CoV-2 infection by recharging glutathione39 and enhancing vitamin D synthesis, among other things. |
| Vitamin C — A number of studies have shown vitamin C can be very helpful in the treatment of viral illnesses, sepsis and ARDS,40 all of which are applicable to COVID-19. Its basic properties include anti-inflammatory, immunomodulatory, antioxidant, antithrombotic and antiviral activities. At high doses, it actually acts as an antiviral drug, actively inactivating viruses. Vitamin C also works synergistically with quercetin.41 |
| Quercetin — A powerful immune booster and broad-spectrum antiviral, quercetin was initially found to provide broad-spectrum protection against SARS coronavirus in the aftermath of the 2003 SARS epidemic,42,43,44 and evidence suggests it may be useful for the prevention and treatment of SARS-CoV-2 as well. |
| B vitamins — B vitamins can also influence several COVID-19-specific disease processes, including45 viral replication and invasion, cytokine storm induction, adaptive immunity and hypercoagulability. |
Mikovits also recommends Type 1 interferons.
“The type 1 [interferon] — the primary source of interferon, alpha and beta — is the plasmacyte dendritic cell. We know that’s dysregulated in people with HIV, with XMRVs, with aberrant retroviral expression. Those people can’t make interferon.
Type 1 interferons can be provided in a spray that you can spray directly into your throat, your nose, and that will give you the protection you need so that the virus doesn’t [replicate]. It degrades it right away … Should you feel cough or fever, headache, immediately up your Type 1 interferon. Take a couple of sprays of that per day prophylactically as well, and that will keep the viral load down.
We know [SARS-CoV-2] isn’t a natural virus, we know this is lab-created, but it’ll calm the expression, it’ll degrade the RNA for those who can’t degrade the RNA, and that’s the job of Type 1 interferon — to have your macrophages be these little Pac-Men that simply degrade the RNA.”
Nebulized Peroxide — My Favorite Treatment Choice
My personal choice for the treatment of COVID-19 symptoms is nebulized peroxide. It’s a home remedy I recommend everyone familiarize themselves with, as in many cases it can improve symptoms in mere hours. You can also use it as a preventive strategy if you know you’ve been exposed to someone who is ill.
Nebulizing hydrogen peroxide into your sinuses, throat and lungs is a simple, straightforward way to augment your body’s natural expression of hydrogen peroxide to combat infections and can be used both prophylactically after known exposure to COVID-19 and as a treatment for mild, moderate and even severe illness.
Dr. David Brownstein, who has successfully treated over 100 COVID-19 patients with nebulized peroxide, published a case paper46 about this treatment in the July 2020 issue of Science, Public Health Policy and The Law. He also reviews its benefits in “How Nebulized Peroxide Helps Against Respiratory Infections.”
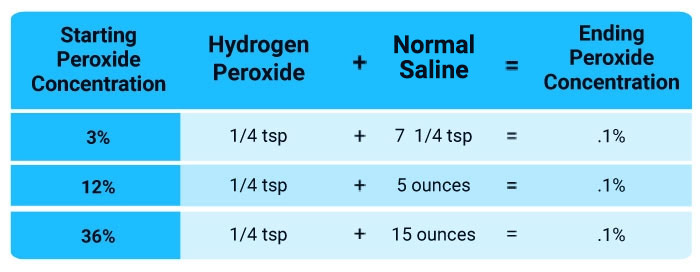
Nebulized hydrogen peroxide is extremely safe, and all you need is a desktop nebulizer and food-grade hydrogen peroxide, which you’ll need to dilute with saline to 0.1% strength. I recommend buying these items beforehand so that you have everything you need and can begin treatment at home at the first signs of a respiratory infection.
In the video above, I go over the basics of this treatment. Be sure to buy a nebulizer that plugs into an electrical outlet, as battery-driven ones are too low-powered to be truly effective. Also make sure your nebulizer comes with a face mask, not just a mouth piece. If it doesn’t come with a face mask, you can pick one up separately. Just search Amazon for “nebulizer face mask for adults.”
– Sources and References
- 1 U.S. CDC December 18, 2020
- 2 Anthrax Vaccine January 11, 2021
- 3, 26, 27 Science December 21, 2020
- 4, 10 Children’s Health Defense August 6, 2020
- 5 Anthrax Vaccine Blog Spot January 11, 2021
- 6 Barron’s April 4, 2020
- 7, 8 STAT News January 10, 2017
- 9, 11, 12, 13, 14, 15 SEC.gov November 9, 2018
- 16, 20 ICAN Letter to Dr. Peter Marks, January 5, 2020
- 17, 18 Pharm Nanotechnol. 2018;6(2):81-93. doi: 10.2174/2211738506666180611100416
- 19 PETITION FOR ADMINISTRATIVE ACTION REGARDING CONFIRMATION OF EFFICACY END POINTS AND USE OF DATA IN CONNECTION WITH THE FOLLOWING CLINICAL TRIAL(S)
- 21 Johns Hopkins December 17, 2020
- 22 FDA Briefing Document December 10, 2020
- 23, 24, 25 FDA VRBPAC Meeting Presentation December 17, 2020
- 28 Anal. Chem. 2016, 88, 23, 11804–11812
- 29, 30 The Defender December 21, 2020
- 31, 33, 34 Forbes July 29, 2020
- 32 Reuters July 23, 2020
- 35 “Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in Patients with the Novel Coronavirus Infection”
- 36 International Journal of Infectious Disease 100 (November 2020): 343-49
- 37 PLoS Pathog. 2010 Nov 4;6(11):e1001176. doi: 10.1371/journal.ppat.1001176.
- 38 “Zinc Fact Sheet for Health Professionals,” U.S. Department of Health & Human Services, National Institutes of Health, updated July 15, 2020
- 39 Neuro Endocrinology Letters 27, no. 3 (June 2006): 365-8
- 40 JAMA 2019 Oct 1; 322(13): 1261–1270
- 41 Frontiers in Immunology June 19, 2020 DOI: 10.3389/fimmu.2020.01451
- 42 Journal of Virology Sep 2004, 78 (20) 11334-11339, Antiviral activity of an analog of luteolin
- 43 Bioorg Med Chem. 2006 Dec 15;14(24):8295-306
- 44 Maclean’s February 24, 2020
- 45 Maturitas August 15, 2020 DOI: 10.1016/j.maturitas.2020.08.007 [Epub ahead of print]
- 46 Science, Public Health Policy and The Law July 2020; 1: 4-22 (PDF)
___
https://articles.mercola.com/sites/articles/archive/2021/02/10/nanoparticles-in-moderna-vaccine.aspx
