FDA Panel Unanimously Endorses J&J Covid Vaccine
ZeroHedge.com
While it was so widely expected the announcement was merely a formality, just after 5pm on Friday the FDA Advisory Panel voted unanimously (22-0) to endorse the (one-shot, no mRNA) J&J vaccine, saying the benefits outweigh the risks, and recommended the agency grant emergency authorization, moving the nation’s third vaccine one step closer to getting into Americans’ arms.
The vaccine was 66% effective in protecting any cases of moderate to severe illness. It was 85% effective against severe cases of COVID-19 and completely prevented hospitalizations and death, four weeks after inoculation.
The FDA could now give the green light to the single-dose vaccine as early as Saturday, and it probably will.
Vaccinations will then begin as soon as a Centers for Disease Control and Prevention (CDC) panel recommends the vaccine and the CDC accepts that recommendation. The CDC panel is scheduled to meet Sunday.
“We are at the precipice of having another vaccine in our toolbox,” CDC Director Rochelle Walensky said Friday. “Having an additional safe and effective vaccine will help protect more people faster.”
The Johnson & Johnson (J&J) vaccine is different from the other two already on the market and could be a potential game changer. It is administered in a single dose, and does not need to be frozen when shipped and stored. It is also not based on the highly controversial mRNA technology used by Pfizer and Moderna. Unlike those two, the JNJ vaccine is what’s called a viral vector vaccine.
To create this vaccine, the Johnson & Johnson team took a harmless adenovirus – the viral vector – and replaced a small piece of its genetic instructions with coronavirus genes for the SARS-CoV-2 spike protein.
After this modified adenovirus is injected into someone’s arm, it enters the person’s cells. The cells then read the genetic instructions needed to make the spike protein and the vaccinated cells make and present the spike protein on their own surface. The person’s immune system then notices these foreign proteins and makes antibodies against them that will protect the person if they are ever exposed to SARS-CoV-2 in the future.
The adenovirus vector vaccine is safe because the adenovirus can’t replicate in human cells or cause disease, and the SARS-CoV-2 spike protein can’t cause COVID–19 without the rest of the coronavirus.
“We need vaccines that are effective and well-tolerated. And importantly, ones that are simple to deploy,” said Gregory Poland, director of the Mayo Clinic’s vaccine research group, who spoke to the panel as part of J&J’s presentation.
The endorsement from the FDA panel of experts comes as politically motivated federal officials are again scrambling to boost the panic meter by warning about the impact of recent highly contagious variants of the coronavirus, urging people not to grow complacent despite plunging cases and hospitalizations. The rise of variants makes vaccination more important than ever, CDC officials said.
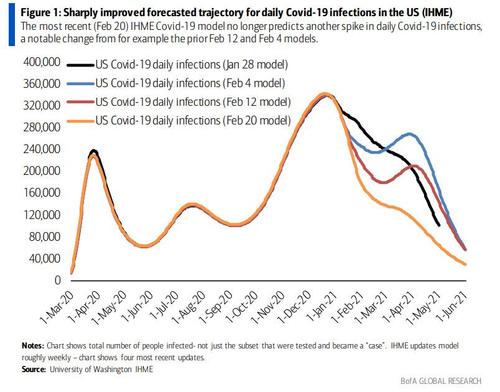
CDC epidemiologist Adam MacNeil told the FDA panel said he expects the B.1.1.7 variant, first found in the United Kingdom, has likely spread throughout the entire U.S., and could become the dominant virus in mid-to-late March. However, inadequate genetic sequencing means we may never get the true picture. Furthermore, recent computer models have predicted that not even covid variants will prevent the US from basically being covid free by June.
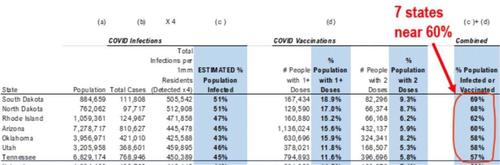
While the pace of vaccinations has been steadily increasing, MacNeil said the U.S. is still “nowhere close” to herd immunity, which also is a politically-motivated falsity because as we showed earlier, at least 7 states are now on the verge of herd immunity. He emphasized the effectiveness of current strategies including masking and physical distancing.
Continuing with the political narrative, to date supply has been the primary constraint to the ramping up of the U.S. vaccination effort. A vaccine by U.S. pharmaceutical giant Pfizer and its German partner BioNTech, and another by Moderna were both authorized in December, but Pfizer has only shipped approximately 40 million doses, while Moderna has shipped about 45 million doses.
Johnson & Johnson’s vaccine will only be available at a relative trickle at first. The company will only have about 4 million doses available to ship immediately upon authorization, but that number will increase to 20 million by the end of next month.
J&J has a deal with the U.S. government to supply 100 million doses of its vaccine by the end of June, and White House COVID-19 coordinator Jeff Zients said this week the federal government will do “everything we can” with the company to ramp up production.
The company asked the FDA to authorize the use of the vaccine in people aged 18 and older, but there were some concerns over the lack of data on recipients older than 75. There were also concerns over the effectiveness in people over the age of 60 with certain pre-existing conditions, like obesity and diabetes.
The J&J vaccine hasn’t been tested yet in children and teens under the age of 18, so it was not authorized for their use. A trial to study the safety and efficacy of the company’s vaccine in teens aged 17 and younger will begin late next month or early April.
Finally, while the two coronavirus vaccines already on the market may appear to be more effective than Johnson & Johnson’s, experts say it is difficult to compare them head-to-head because of different clinical trial designs and different endpoints. Furthermore, the fact that the two previous vaccines are mRNA based – a rather novel and untested technology – may have sparked skepticism among many Americans.
“I really think we need to be careful not to read into the data, to look across studies when they are so different, and instead look at each vaccine individually,” CDC’s Nancy Messioner said Friday during an interview with the Journal of the American Medical Association.
___
https://www.zerohedge.com/medical/fda-panel-unanimously-endorses-jj-covid-vaccine