FDA Panel Rejects Broad Pfizer Booster Jabs Scuttling Biden’s Plan, Endorses Limited Shots For “At Risk” Elderly
ZeroHedge.com
Update (1620ET): Minutes after the FDA advisory panel rejected the Biden Administration’s plan to dole out booster shots to patients as young as 16, the same panel returned and voted unanimously to temporarily approve Pfizer Booster jabs for patients who are either a.) 65 or older, b.) immunocompromised or c.) both.
Ultimately, the panel voted 18-0 to support an emergency-use authorization (a more limited clearance than a full approval sought by the administration and Pfizer) for people 65 and older or individuals at high risk of severe COVID.
Ahead of the vote, analysts at several Wall Street banks and research shots told clients a limited authorization would be the most likely outcome amid a groundswell of opposition for a broad booster dose.
As one Twitter user pointed out, the decision represents the biggest bureaucratic revolt against the Biden Admin’s COVID strategy yet.
As a reminder, today’s public hearing clearly revealed that scientists can have conflicts of interest, too.
Remember, although the FDA isn’t obligated to follow the advisory panel’s decision, it typically does. A final decision will likely arrive next week.
Now, we wait to see what a similar advisory panel at the CDC decides next week as well.
* * *
Update (1530ET): A panel of senior advisors for the FDA voted against approving a third dose of the Pfizer-BioNTech jab for every patient over the age of 16, while leaving a door open to approving booster jabs for a more limited portion of the population including the elderly and the immuno-compromised.
The final vote tally was Yes: 2, No: 16, Abstain: 0.
The committee was charged with voting whether the safety and effectiveness data from Pfizer’s clinical trial supported approval of the company’s booster dose among people 16 years and older. The meeting included members of the FDA’s Vaccines and Related Biological Products Advisory Committee, as well as officials from the CDC, Israel’s Health Ministry, vaccine experts and Pfizer representative.
During a day of publicly televised debate, some of the advisors indicated that while they see a potential need for older or other more vulnerable patients o receive a third dose, the need for younger people could be less pressing, and may not be offset by the risks of dangerous side effects.
“There are very clear populations where a booster may be appropriate, such as elderly or immunocompromised,” said National Institutes of Health scientist Michael Kurilla. “It’s not clear to me that the data we’re seeing right now is applicable or necessary for the general population.”
At one point, panelist Paul Offit questioned the wide framing of the FDA question, and raised concerns about the risk of myocarditis, a kind of heart inflammation, in younger men (for more on that see here).
As CNBC’s Meg Tirrell reports, with advisors now considering a modified question, this might not be the final vote of the day.
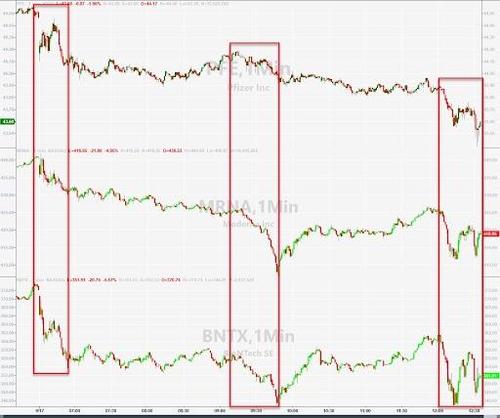
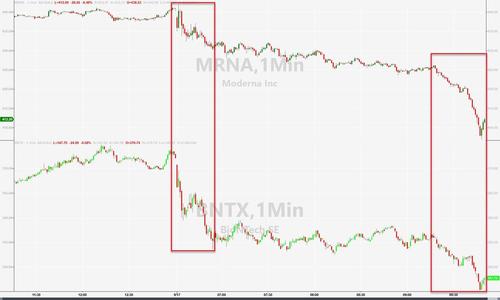
Stocks of vaccine-makers are tumbling on the news.
The results hit just as Dr. Fauci was set to start an interview with the CNBC, where he was left twisting in the wind, insisting that he was totally unsurprised by the FDA’s decision not to rubber-stamp the Biden Administration’s non-science-based plan, while acting like he and the rest of the admin’s team wasn’t pushing for approval all along.
Pressed to explain Israel’s ongoing surge, which booster jabs have been unsuccessful in stamping out, Dr. Fauci droned on incoherently about the science behind “breakthrough infections”, while arguing that Israel is doing a better job of counting asymptomatic infections, thereby inflating its infection numbers (though he apparently overlooked the fact that US is guilty of over-testing itself).
In the latest sign that the media is intensifying its scrutiny of booster jabs, CNBC’s Sara Eisen didn’t go easy on Dr. Fauci.
To be clear, the role of the FDA’s advisory panel in this case has to determine whether an additional dose can be used, whereas an expert panel advising the CDC (Advisory Committee on Immunization Practices, or ACIP), will convene Wednesday to offer its own recommendation on booster jabs.
Meanwhile, it doesn’t look like Biden’s plan to offer booster jabs to all comers as of Monday has been delayed – at least for now.
According to Dr. Fauci, the big question now will be “at what age” will the advisory board recommend boosters – “will it be 60, 50 40…”.
The committee is now taking a brief break to revise the question and perhaps narrow it to cover people over age 65 and those with immunocompromised issues.
* * *
Update (1255ET): As The FDA Panel hears from various health officials during the public comment section on the pros and cons of a booster shot, some very uncomfortable facts are coming out about vaccine efficacy and vaccine side effects – facts that anywhere else would immediately be deplatformed and banned from discourse.
Perhaps this is why two top FDA officials just resigned.
“Current evidence does not, therefore, appear to show a need for boosting in the general population, in which efficacy against severe disease remains high,” FDA scientists Marion Gruber and Phil Krause wrote.
“If unnecessary boosting causes significant adverse reactions, there could be implications for vaccine acceptance that go beyond COVID-19 vaccines. Thus, widespread boosting should be undertaken only if there is clear evidence that it is appropriate,” the scientists said.
And then there was this slide…
Pfizer shares are sliding but Moderna and BioNtech stock prices are plunging as Evercore ISI analyst Umer Raffat predicts that the panel of advisers won’t recommend booster shots (with the vote due later today)…
Raffat in a midday note Friday says voting members on the advisory committee “are already leaning against boosters” and the “FDA does not sound convinced by balance of evidence to date”
The CDC advisory group now says it will take up the need for booster shots at a meeting now scheduled for Sept 22nd and Sept 23rd. That makes it all but certain no booster rollout will happen Monday as the President gave as a date to possibly get boosters.
* * *
The big day has finally arrived.
On Friday, a group of key FDA advisors is meeting to debate and vote on Pfizer’s request for approval of a third COVID booster shot of its “Comirnaty” vaccine for all Americans age 16 and older. The two big questions they must answer are: is there enough evidence to suggest that booster shots are safe and should be made available to everyone, or should they be limited to a smaller group of older and immunocompromised Americans?
On the agenda are presentations from Pfizer, FDA staff, CDC staff, Israeli researchers, and others. At the end of the virtual meeting, which began at 0830ET Eastern, members will be asked to vote yes or no. That vote isn’t expected until later in the afternoon after a long day of debate.
Interested parties can watch the debate streaming live via YouTube:
After the White House waffled on whether the shot could be delivered five or eight months after the second dose, Pfizer has requested approval for the booster dose about six months after the second shot after submitting data showing efficacy wanes over time. And while Moderna has made a similar request and submitted similar data, it’s vaccine isn’t being considered on Friday. A committee of advisors from the CDC will meet next week to develop booster shots. Despite the drug companies’ best efforts, the FDA has seemingly remained unconvinced about the necessity of booster jabs.
In recent weeks, scientists have become increasingly vocal about their opposition for the US pushing ahead with booster jabs so soon. Most argue that these jabs would be better utilized in the developing world, where vaccination rates are lower, and the risk of a deadly new variant arising are higher. One exasperated scientist lamented that the scientific process was being “short circuited” by politics, a reference to President Biden’s push to start doling out booster jabs in the face of the delta driven wave that surged over the summer and – in the US, at least – appears to have finally peaked (with deaths hopefully soon to follow). The WHO has also urged President Biden to hold off on the booster jabs, arguing that there’s greater need elsewhere.
University of Florida biostatistician Ira Longini (a co-author on the Lancet paper we will mention below) said it would be “immoral” to begin widespread boosters before the rest of the world has been vaccinated, per CNN.
On the data front, new data published this week out of Israel offer a “compelling” case for a booster to be administered at some point as vaccines-induced efficacy wanes. But the data is based on a sample of just 300 patients.
Meanwhile, on Monday, an international group of scientists led by Dr. Philip Krause, deputy chief of the FDA’s vaccine regulation office, and his boss, Dr. Marion Gruber – both of whom are leaving the FDA, apparently in protest, published an essay in The Lancet that questioned the need for widespread booster shots right now. Gruber, who has decided to remain at the agency until later this fall, is listed as a participant in Friday’s meeting, .
In a nutshell, the Lancet paper argues that vaccine-based protection against severe Covid is still strong, while evidence is lacking that booster shots will be safe and effective.
So far, the real-world data is scant and inconclusive. While Israel has doled out hundreds of thousands of booster jabs to patients as young as 12, it hasn’t done much to stop the latest wave of cases (like in the US, deaths and hospitalizations are much lower than their 2020 peak).
Right now, evidence suggests boosters would have the greatest benefit for patients over 65, and the immunocompromised. Already, 1.9MM Americans have received booster jabs after they were authorized by the CDC for people with compromised immune systems.
Shares of vaccine-makers including Pfizer, BioNTech, Moderna and others could be volatile Friday as advisers meet, and inevitable leaks hit the tap.
“My guess is we are going to end up with a recommendation for booster doses for a certain subpopulation, such as adults older than 65,” said Bill Moss, executive director of the International Vaccine Access Center at the Johns Hopkins Bloomberg School of Public Health.
Before we go, let’s review: The FDA (and plenty of advisers and third parties) say vaccines are largely still effective enough that there’s no need for boosters. Pfizer has submitted data showing efficacy is waning, and that boosters are necessary. Dr. Fauci, and seven other top health officials including the heads of the CDC and FDA have spoken in favor of the need for booster jabs. The WHO and plenty of private scientists have insisted instead that those jabs should instead go to the emerging world where vaccination rates are much lower. For what it’s worth, when confronted about natural vs. vaccine-induced immunity, Dr. Fauci said “I really don’t know what to tell you.”
But most importantly: the White House says ‘GET YOUR BOOSTER JABS NOW!’ with President Biden pushing ahead with a plan to start doling out the jabs on Monday (contingent (though that plan must receive the blessing of the FDA and CDC first).
The bottom line is this: while the White House, Dr. Fauci and every other party involved claim to be following “the science”, but the reality is the science hasn’t really given us a clear answer. It’s clear that vaccine-induced immunity fades over time – and generally more quickly than natural immunity – but as for whether a third dose will make a material difference in preventing death and serious illness in the vast majority of patients? Scientists still haven’t collected and analyzed enough data.
The bigger question: will ‘Uncle Joe’ and myriad state and local officials require Americans to get booster jabs like they’re requiring even those with natural immunity to get vaccinated, potentially exposing these patients to rare side effects while it’s unclear whether they’re making a meaningful difference in immunity.
The panel is meeting between 1425 and 1625. It’s likely the decision won’t arrive until after the market close. We will update readers with the result once it’s finally released.
Read the agenda below:
Agenda on Scribd
___
https://www.zerohedge.com/covid-19/fda-advisors-meet-decide-booster-jabs-science-remains-uncertain