FDA Committee Discussion Opines Men Under 40 at Greater Risk of Vaccine-Related Myocarditis than COVID Itself.
ZeroHedge.com
Following the release of the Biden administration’s six-step plan to increase vaccination rates across the country, it seemed that their charge toward a de facto national vaccine mandate had reached terminal velocity. Yet, in advance of the administration’s decision to green light booster shots to address the waning efficacy of vaccines, their steadfast effort reached unexpected resistance from an even more unexpected source. On September 18, the FDA declared that its authorization for the expansion of booster shots beyond the immunocompromised would only apply to persons 65 years or older. The FDA committee which authorized the administration of boosters for that 65+ demographic by a 18-0 vote issued an equally resounding vote against approving the measure for people below 65 by a vote of 16-3.
At best, the decision was made at a time where data on the efficacy of boosters does not clearly support policy making expanding their authorization. When pressed on that very issue, CDC Director Rochelle Wallensky didn’t offer a resounding endorsement when she stated that “so there’s actually hope, we don’t have data yet.” Another less than stellar endorsement of expanding boosters came from a study published in the New England Journal of Medicine which examined the efficacy of the Israeli government’s booster initiative which began on July 30. While the study determined that the rate of infection was lower among recipients of booster shots, the threshold for the enhanced protection they achieved was only measured at a period of 12 days following the third dosage. Even then, the rate of infection only fell by around 11%. While these instances highlight the uncertainty of the long-term success of the Biden administrations booster initiative, the FDA panel discussion on the issue did highlight one thing with much greater certainty — the risks of vaccination side effects can outweigh the risk of COVID-19 themselves.
In a stark admission which has been drowned out by attempts to make the panels decision reflect well upon the Biden administration, an FDA Center for Biologics Evaluation and Research advisory panel discussion declared that the risk of myocarditis being higher than COVID-19 in males under the age of 40. The assertion was predicated upon public health claims data cited by Dr. Doran Fink of the FDA, who implored Pfizer to take a greater initiative to examine this very issue. Given this profound point of demarcation, this topic of discussion during the FDA advisory panel’s meeting offers greater insight into the limited scope of the authorization of booster shots.
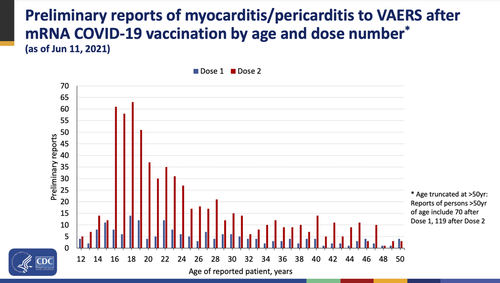
Though the determination of the risk of vaccine-associated myocarditis being greater than COVID-19 itself applied to men under 40, publicly available data from the CDC released in June reflects that young men between 16 and 30 years old accounted for the highest volume of preliminary reports citing this side effect.
Along with pericarditis, myocarditis has been one of the more glaring side effects of the Pfizer-BioNTech COVID-19 vaccine which has been approved by the FDA to be produced under the brand-name Comirnaty. This cause for concern was motive enough for the FDA to add a warning to the vaccines regarding these side effects in June, well-after its initial emergency use declaring them “safe and effective” was issued. As of September 10, cases of myocarditis and pericarditis made up 5,765 of the 701,559 reports of adverse events made to the Vaccine Adverse Event Reporting System (VAERS). Additionally, heart attacks account for another 6,637 adverse events made to VAERS.
According to Pfizer, potential side effects from potential booster shots are likely to occur at a rate similar to those following the second dose of their vaccine. When referring to the aforementioned CDC data set, instances of pericarditis and myocarditis were exponentially more prominent following the second dose of the vaccine than the first. The mounting evidence of the risk of heart-related severe adverse events from all COVID-19 vaccines seems to have been a clear influence on the panels 16-3 vote against authorizing booster shots for those under the age of 65, given the context of the committee discussion on this very issue.
As the Biden administration’s actions permeate into the lives of the millions of Americans who will be impacted by its declaration to have the Department of Labor craft a rule mandating vaccines for employers with a staff of over 100, one must ask how much that conjecture contemplated risks that have impacted the FDA’s own policy making decisions. With a CDC advisory committee set to make its determination on this very issue next week, one can only hope they will give credence to the forewarning.